FAF Products
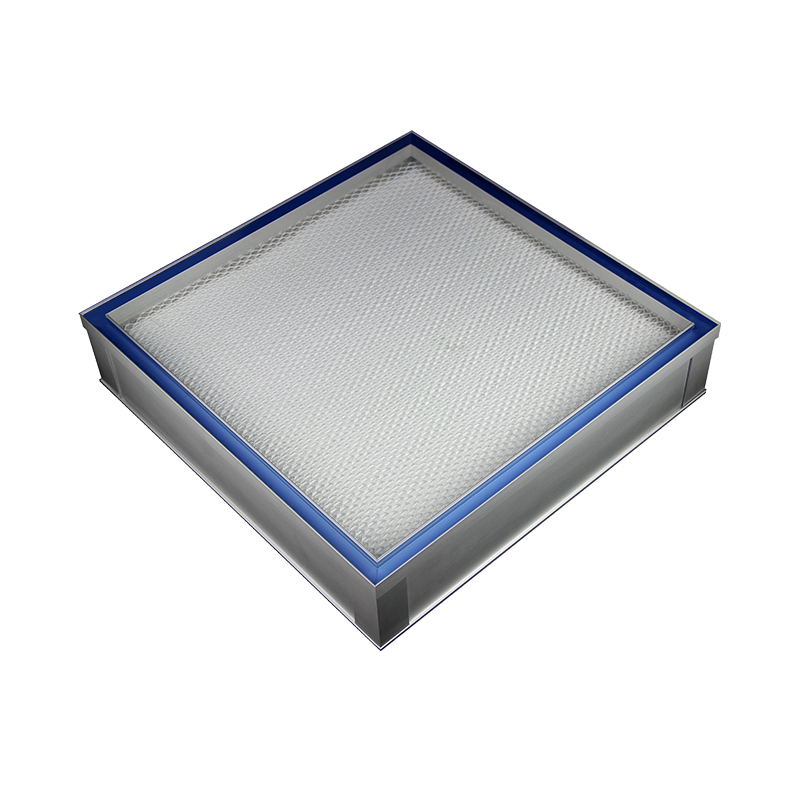
Top Gel Seal Mini-pleat HEPA filter
Product Overview
• Minimum 99.99% at 0.3μm, H13, and 99.995% at MPPS, H14.
• Polyalphaolefin (PAO) compatible.
• Lowest pressure drop mini-pleat HEPA filter available for pharma, life sciences.
• Lightweight galvanized or aluminum or stainless steel’s frame available.
• Gel, gasket, or knife-edge seal available.
• Thermoplastic hot-melt separators.
Typical Applications
• Pharmaceutical
• Life Sciences
• Biosafety
• Healthcare
• Pill Encapsulation
FAF‘s Advantages and Features
FAF’S HEPA filter designed specifically for the unique requirements and challenges of the pharmaceutical industry, the filter has the proven durability, polyalphaolefin (PAO) compatibility, high particulate filtration efficiency.Proven Reliability with Exceptional Performance
Designed to increase cleanroom uptime and reduce the risks associated with pharmaceutical manufacturing.
Pharmaceutical grade microglass, delivering superior performance.
Extremely low off-gassing of chemical components, resulting in the highest quality clean air available.
Lowest pressure drop mini-pleat HEPA filter available, reducing energy consumption for significant savings.
Manufactured, tested, and packaged in ISO 7 clean facilities to ensure the highest purity, quality, and consistency.
Reduce Operational Risk
The pharmaceutical industry estimates that 77% of production downtime can be attributed to failures of equipment and environmental problems,with successful operation requires utilizing HEPA filters with dramatically higher tensile strength that is highly resistant, thereby eliminating premature leaking and failure.
Increase Uptime
While FDA Testing Guidance requires critical room leak-testing certification twice a year . Creasing time between certifications results in less PAO exposure to the gel seal , lower labor costs, and increased production time.
FAQ
1. Can you accept sample orders?
We can.
2. What is your packaging?
Can be packaged according to customers actual requirements if you need.
3. How long is your delivery time
The stock out of the warehouse is 2-5 days, 100 standard products, about 10-15 days.








